Global Journal of Medical and Clinical Case Reports
Tyrosinemia Type 1: A Case Prior to Newborn Screening and the Importance of Early Treatment
Hugo Listo Quílez1, Fernando Hernández García2 and José Manuel López Álvarez3,4*
1Intensive Care Unit, Sant Joan d’Alacant University Hospital, Alicante, Spain
2Intensive Care Unit, Insular University Maternal and Child Complex, Las Palmas de Gran Canaria, Spain
3Pediatric Intensive Care Unit, Insular Maternal and Child University Complex, Las Palmas de Gran Canaria, Spain
4Fernando Pessoa University of the Canary Islands. Santa María de Guía, Las Palmas de Gran Canaria, Spain
Cite this as
Quílez HL, García FH, López Álvarez JM. Tyrosinemia Type 1: A Case Prior to Newborn Screening and the Importance of Early Treatment. Glob J Medical Clin Case Rep. 2025:12(7):143-147. Available from: 10.17352/2455-5282.000215Copyright License
© 2025 Quílez HL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Tyrosinemia type 1 (TYR1) is a severe hereditary metabolic disorder caused by a deficiency of the enzyme fumarylacetoacetate hydrolase (FAH), leading to the accumulation of toxic metabolites and hepatorenal damage. Without treatment, it may progress to liver failure or hepatocellular carcinoma. Nitisinone is the treatment of choice, as it blocks the formation of toxic compounds. Early diagnosis is critical, particularly in contexts where newborn screening is not yet implemented.
Case report: We present the case of a 2-month-old infant, born to consanguineous parents, who presented with abdominal distension, vomiting, and diarrhea. The patient exhibited severe coagulopathy, metabolic acidosis, and ultrasound findings suggestive of acute abdomen. After transfer to a tertiary care center and completion of metabolic studies, a diagnosis of TYR1 was confirmed. Nitisinone therapy was initiated, with a favorable outcome following a complex stay in the intensive care unit.
Discussion: Although the use of nitisinone has significantly reduced the need for liver transplantation, further studies are needed to better understand its potential long-term neurocognitive effects. Continuous clinical and neuropsychological monitoring is recommended for affected individuals.
Conclusions: Early diagnosis of TYR1 through newborn screening enables prompt initiation of treatment with nitisinone and dietary management, significantly improving survival and reducing the risk of hepatocellular carcinoma. While treatment has dramatically changed the prognosis, uncertainties remain regarding long-term effects, particularly at the neurocognitive level. Comprehensive follow-up, including clinical, metabolic, and neuropsychological assessments, is essential.
Abbreviations
TYR1: Tyrosinemia type 1; FAH: Fumarylacetoacetate Hydrolase; FAA: Fumarylacetoacetate; TAT: Tyrosine Aminotransferase; SAA: Succinylacetoacetate; SA: Succinylacetone; HPD: 4-Hydroxyphenylpyruvate dioxygenase
Introduction
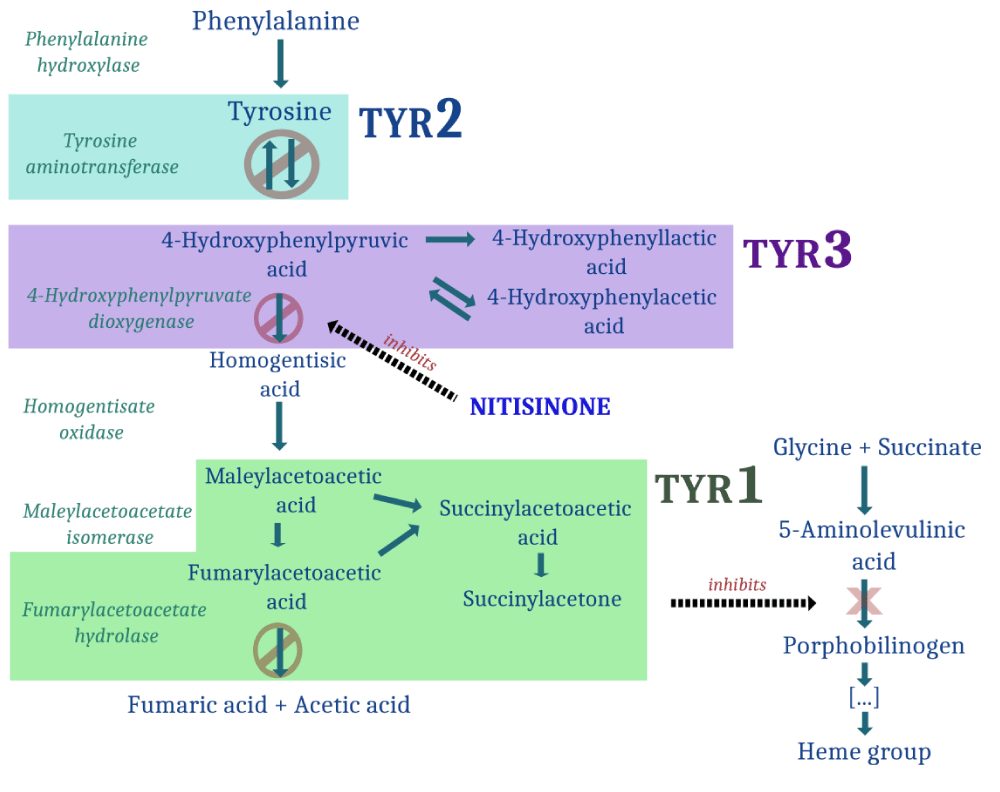
Tyrosinemia type 1 (TYR1) is the most severe hereditary disorder of tyrosine metabolism. It occurs in approximately 1 in 100,000 individuals of Northern European descent. TYR1 is caused by a deficiency of fumarylacetoacetate hydrolase (FAH), the final enzyme in the tyrosine catabolic pathway (Figure 1). Its substrate, fumarylacetoacetate (FAA), accumulates in hepatocytes and proximal renal tubular cells, resulting in progressive hepatic and renal injury.
FAA induces oxidative damage by reacting with glutathione and protein sulfhydryl groups. It is also a potent mutagen, and this mutagenicity is believed to contribute to the high incidence of hepatocellular carcinoma observed in TYR1.
The oxidative and genotoxic effects of FAA result in either cell death or profound dysregulation of gene expression, especially in the liver. Consequently, multiple hepatic metabolic functions are impaired, including gluconeogenesis, ammonia detoxification, and synthesis of secretory proteins such as albumin, clotting factors, complement proteins, lipoproteins, and cytokines. FAH-deficient hepatocytes also exhibit markedly reduced levels of tyrosine aminotransferase (TAT), the first enzyme in tyrosine degradation, leading to elevated plasma tyrosine levels. Although tyrosine is not directly hepatotoxic or nephrotoxic, it is associated with dermatologic, ocular, and potentially neurodevelopmental complications.
FAA has a short intracellular half-life and is undetectable in body fluids of patients with TYR1. However, its downstream metabolites—succinylacetoacetate (SAA) and succinylacetone (SA)—are released into circulation and can be measured for diagnostic purposes (Figure 1).
The first-line medical therapy is nitisinone (formerly NTBC), an inhibitor of 4-hydroxyphenylpyruvate dioxygenase (HPD), an early enzyme in the tyrosine degradation pathway. Notably, a deficiency of HPD causes Tyrosinemia type 3. Nitisinone treatment limits the metabolic flux through the tyrosine pathway, thereby preventing the accumulation of the toxic intermediates FAA and SA (Figure 1) [1].
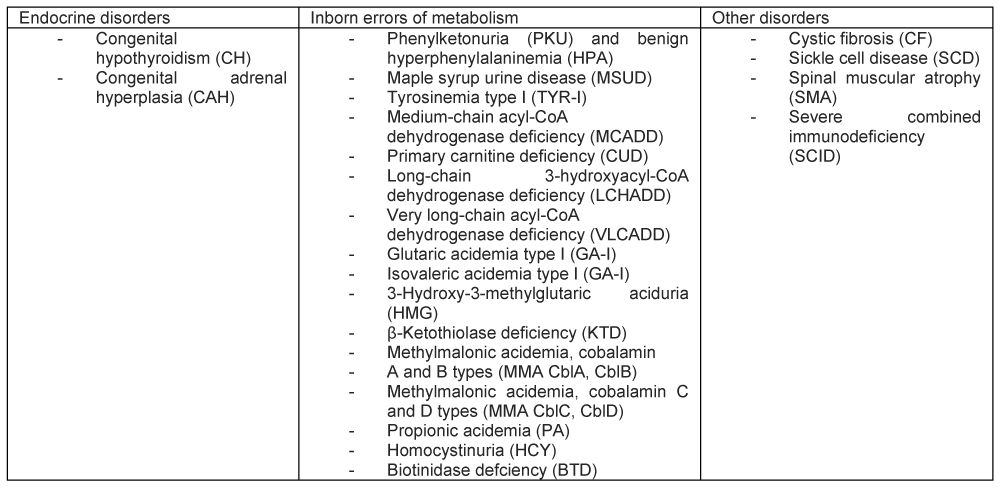
In Spain, TYR1 was approved for inclusion in the national newborn screening program by the Public Health Commission of the Interterritorial Council of the National Health System in 2024 and is currently undergoing regulatory implementation. Disorders with significant clinical benefit from early detection are progressively being added to the newborn screening panel (Figure 2).
Since its initial implementation in 2014, TYR1 has been included in the heel-prick newborn screening panel in some autonomous communities. In the Canary Islands, systematic screening began in 2023 [2].
DISORDERS INCLUDED IN THE SPANISH NATIONAL NEWBORN SCREENING PROGRAM (2025)
We report a case of TYR1 diagnosed in 2021, prior to the implementation of systematic screening.
Our aim is to describe the clinical presentation of this rare disorder, which has highly variable manifestations, and to highlight the importance of early diagnosis and prompt initiation of treatment.
Case Presentation
We present the case of a male infant born in 2021 at 39+4 weeks of gestation, with a birth weight of 3200 g. He was the fourth child of healthy, consanguineous parents (second cousins), and the pregnancy and delivery were uneventful. The infant was exclusively breastfed.
At 75 days of age, he was brought to the emergency department of a regional hospital in the Canary Islands due to a 3-day history of foul-smelling, watery diarrhea without blood or mucus, associated with abdominal pain and irritability. Since birth, his parents had noted daily episodes of voluminous vomiting, constipation, and abdominal discomfort.
Initial assessment and investigations
On examination, the infant appeared moderately ill with a markedly distended, firm, and tender abdomen. Laboratory findings revealed anemia (hemoglobin 9.9 g/dL), leukocytosis (17,000/µL), severe coagulopathy (Quick time 8.0%, INR 8.13, APTT 76.5 s), and lactic acidosis (pH 7.18, lactate 8.3 mmol/L). Liver function tests showed a direct bilirubin level of 1.87 mg/dL (total bilirubin 3.0 mg/dL), near-normal transaminases (ALT 27 U/L, AST 88 U/L), GGT 106 U/L, LDH 584 U/L, and normal procalcitonin and CRP levels. Coagulation studies revealed a global factor deficiency, sparing factor VIII.
An urgent abdominal ultrasound suggested possible ileocolic intussusception in the right iliac fossa, with lymphadenopathy at the lead point, paralytic ileus, and free peritoneal fluid.
Surgical findings
Emergency exploratory laparotomy ruled out intussusception but revealed edematous bowel loops, multiple enlarged abdominal lymph nodes (> 1.5 cm), and granuloma-like hepatic lesions.
Hospital course
The infant remained hemodynamically and respiratorily stable. He received fluid resuscitation (10 mL/kg crystalloids), one unit of fresh frozen plasma, vitamin K (1 mg), and red blood cell transfusion for anemia (nadir Hb 7.6 g/dL). Empirical antibiotics were started (cefotaxime, vancomycin, metronidazole). Given the clinical picture, both abdominal sepsis and an inborn error of metabolism were considered, prompting transfer to a tertiary center within 24 hours.
Metabolic and genetic workup
Upon admission, metabolic acidosis persisted, requiring intravenous 1/6 molar sodium bicarbonate. Urinary organic acid analysis showed elevated levels of 4-hydroxyphenyllactic, 4-hydroxyphenylpyruvic, 4-hydroxyphenylacetic, β-phenyllactic, and β-phenylpyruvic acids. Succinylacetone (SA) was markedly elevated at 178 mmol/mol creatinine. Plasma tyrosine was 131 µmol/L (normal 35 – 101), and methionine was elevated at 136 µmol/L (normal 18 – 36). Genetic testing confirmed a homozygous pathogenic FAH variant (c.554-1G>T), establishing the diagnosis of hereditary tyrosinemia type 1 (TYR1).
Nitisinone was initiated at 2 mg/kg/day and later adjusted based on plasma levels.
Pediatric ICU Course (36 Days)
Neurologic: Stable, without pathological signs.
Hemodynamic: Initially required low-dose norepinephrine and dobutamine (< 48 h) to optimize diuresis.
Respiratory: Managed with high-flow nasal cannula (10 – 12 L/min, FiO₂ 30%). On PICU day 14, developed nosocomial pneumonia requiring brief intubation and mechanical ventilation, with rapid improvement.
Metabolic: Nitisinone and Carbaglu (initially 100 mg/kg/day) were started. Acid-base balance was challenging, requiring intermittent 1M bicarbonate boluses and continuous 1/6 M infusion.
Nutrition: Total parenteral nutrition met 100% of basal needs, with 0.5 – 1 g/kg/day of protein and 1 g/kg/day of lipids. Enteral feeding via transpyloric tube with a tyrosine-free formula was introduced. At discharge, he tolerated 45 – 50 mL of oral feeds every 3 hours, supplemented with nasogastric feeding (total intake: 111 kcal/kg/day, 2 g/kg/day protein).
Renal: Initially oliguric with preserved function, likely prerenal. Post-surgery, developed portal hypertension, tense ascites, and abdominal compartment syndrome (intra-abdominal pressure up to 28 mmHg), requiring daily paracentesis for 14 days. Acute kidney injury (peak creatinine 0.97 mg/dL) resolved with brief vasoactive support. Renal function normalized by discharge.
Hematologic/Coagulopathy: Received multiple transfusions (532 mL red cells, 100 mL platelets, 1200 mL plasma). Coagulation factors improved, but hepatic-synthesized factors remained low with prolonged clotting times.
Infectious: Initial ascitic fluid culture yielded E. coli, prompting de-escalation to cefotaxime. On day 19, inflammatory markers rose without identified pathogen, treated empirically with piperacillin-tazobactam. Later developed nosocomial pneumonia treated with meropenem and amikacin. Rhinovirus was detected in nasopharyngeal aspirate. Cytomegalovirus (CMV) viremia and viruria were treated with ganciclovir for 6 weeks.
Additional investigations
- Echocardiogram: Mild aortic insufficiency; pulmonary trunk enlargement without gradient.
- Cranial ultrasound & evoked potentials: Normal.
- Abdominal imaging: Admission ultrasound revealed hepatomegaly with nodular borders, multiple hyperechoic lesions, ascites, and bowel wall thickening. Post-decompression imaging showed improvement. At discharge, liver remained enlarged with persistent hypoechoic nodules (largest: 20 mm in segment VIII), splenomegaly (65 mm), and minimal ascites.
- Transfontanellar ultrasound: Unremarkable.
Follow-up
After discharge, the patient remained clinically stable on nitisinone and a tyrosine-free diet. Unfortunately, follow-up was lost 10 months later due to family relocation abroad.
Discussion
Early diagnosis of TYR1 is critical to improving long-term outcomes. Prompt detection enables early initiation of treatment with nitisinone and a tyrosine-restricted diet, which significantly increases survival without the need for liver transplantation and reduces the risk of hepatocellular carcinoma—particularly when treatment begins before the onset of symptoms [2].
The relevance of early detection is well illustrated in a case series involving two siblings with TYR1. The older child, diagnosed late after presenting with life-threatening complications including hypertrophic cardiomyopathy and hepatic dysfunction, required intensive care but improved with nitisinone. In contrast, the younger sibling was identified through selective newborn screening and started treatment during the first week of life, remaining completely asymptomatic during follow-up. This comparison underscores how early intervention can prevent the clinical manifestations of TYR1 [3].
A growing body of literature supports the early initiation of nitisinone therapy, demonstrating substantial benefits in reducing morbidity and mortality. Classic complications such as acute liver failure, renal tubular dysfunction, neurologic crises, rickets, and early-onset hepatocellular carcinoma can be dramatically reduced—or even prevented—when treatment begins within the first month of life. This level of early intervention is only feasible with effective newborn screening programs that detect succinylacetone (SA) in blood [4].
A particularly illustrative case is presented by Imseis, et al. in a case report of a 7-year-old girl diagnosed with TYR1 in an area where newborn screening was not yet implemented. By the time of diagnosis, the patient had already developed severe complications, including chronic kidney disease, myopathy, rickets, and hepatocellular carcinoma. Although treatment with nitisinone led to partial biochemical improvement, she ultimately required liver transplantation. In contrast, data from screened populations show that infants who initiate nitisinone treatment within the first month of life do not develop liver lesions during at least five years of follow-up and rarely require transplantation. This case clearly reinforces the role of early screening in altering the natural history of TYR1 [5].
Since the introduction of nitisinone, liver transplantation—which was once the only curative option—has been reserved for patients with confirmed malignancy, decompensated liver failure unresponsive to medical therapy, or lack of access to pharmacologic treatment. The aim of transplantation is both functional (restoration of hepatic metabolism) and preventive (eliminating the risk of malignant transformation). According to UNOS registry data, liver transplantation in this context yields favorable outcomes, with one- and five-year survival rates of 90.4%.
Even after transplantation, plasma and urinary SA levels may remain mildly elevated, likely due to persistent extrahepatic production, particularly by the kidneys. The long-term significance of this residual excretion is uncertain, although there have been concerns about potential renal complications such as tubulopathies or renal tubular acidosis. Nevertheless, these associations remain unproven. Importantly, untreated TYR1 commonly manifests with a Fanconi-like renal syndrome, including aminoaciduria, glucosuria, phosphaturia, and acidosis.
While early treatment reduces the incidence of hepatocellular carcinoma in children, it remains unclear whether the same protective effect extends to patients treated in adulthood. Furthermore, long-term consequences of chronic tyrosinemia induced by nitisinone therapy are not fully understood. Concerns have been raised regarding the neuropsychological and behavioral effects of sustained high tyrosine levels. Despite these uncertainties, the introduction of nitisinone, in combination with dietary control, has transformed TYR1 from a fatal infantile disease into a manageable chronic condition with significantly improved outcomes.
Pharmacologically, nitisinone is a triketone compound originally developed as an herbicide. Its mechanism of action was discovered during toxicological studies in rodents, which showed elevated plasma tyrosine and corneal lesions. These findings led to the identification of nitisinone as a potent inhibitor of HPD, effectively blocking a key upstream step in tyrosine catabolism. By doing so, it prevents the accumulation of toxic downstream metabolites, including fumarylacetoacetate and succinylacetone. The first clinical trial in 1992 demonstrated rapid and significant improvement in five patients, laying the foundation for current therapeutic protocols [4].
Today, plasma levels of nitisinone can be monitored and adjusted to maintain effective concentrations (typically 40–60 µM), optimizing metabolic control [4]. Clinical trials have confirmed its impact: a 2007 international study involving 207 patients showed significantly improved survival with nitisinone and diet compared to dietary management alone. Among those treated before 2 months of age, four-year survival reached 88%, compared to 29% in untreated cohorts. Adverse effects were uncommon and generally reversible, including mild hematologic and ocular symptoms [6].
Nevertheless, the potential long-term neurological effects of nitisinone remain a subject of ongoing investigation. This concern stems in part from reports of neurological complications in patients with hereditary HPD deficiency—the same enzyme targeted by nitisinone [1].
Several recent studies have explored the neuropsychological profile of TYR1 patients on nitisinone. Emerging evidence suggests a higher-than-expected incidence of cognitive impairments, including reduced IQ, learning disabilities, attention deficits, and behavioral issues. However, these findings are based on heterogeneous data, often limited to IQ testing, and should be interpreted cautiously. Still, they highlight the importance of including neurodevelopmental monitoring in the long-term follow-up of these patients [4].
In a 2016 study published in Orphanet Journal of Rare Diseases, TYR1 patients treated with nitisinone and diet were compared to healthy controls. The TYR1 cohort demonstrated lower estimated IQ and deficits in executive functioning and social cognition. Notably, there was a negative correlation between the duration of nitisinone therapy and cognitive outcomes. These findings raise the possibility of either a direct neurotoxic effect of nitisinone or the indirect consequences of sustained tyrosinemia. The authors concluded that comprehensive neuropsychological evaluations—not just IQ—are necessary for adequate monitoring and called for further studies to clarify the underlying mechanisms [7].
Conclusion
The present case illustrates the severe clinical consequences of Tyrosinemia type 1 (TYR1) when diagnosis is delayed. Late identification—as in this patient, diagnosed in 2021 prior to the systematic implementation of newborn screening in his region—is associated with a more complex clinical course and a heightened risk of hepatic and neurological complications.
The implementation of universal newborn screening has been instrumental in improving survival and preventing life-threatening complications. We advocate for the expansion of perinatal screening programs to all countries where they are not yet in place, and emphasize the importance of maintaining a high index of clinical suspicion in settings without established screening, given the potentially variable clinical presentation of affected individuals.
However, questions remain regarding the long-term effects of treatment, particularly with respect to neurocognitive development. Current evidence suggests that elevated tyrosine levels—resulting from nitisinone therapy—may adversely impact cognitive performance, highlighting the need for longitudinal studies to more precisely determine the long-term effects of treatment.
Despite these limitations, the combined use of nitisinone and dietary restriction has radically transformed the management of TYR1, converting what was once an almost universally fatal pediatric disorder into a manageable chronic condition. Continued research into the underlying pathophysiological mechanisms remains essential to refine therapeutic strategies and improve long-term quality of life for affected patients. A multidisciplinary approach, including routine clinical, biochemical, and neuropsychological assessments, should be an integral part of long-term follow-up in these patients.
Declarations
ChatGPT (OpenAI) was used to improve the language and clarity of the manuscript by rephrasing selected paragraphs and assisting with English translation. The final content was reviewed and approved by the authors.
Informed consent and conflict of interest
Informed consent was obtained from the patient’s parents for the use of clinical information for research and scientific publication purposes.
- Grompe M. Disorders of tyrosine metabolism. In: Connor RF, editor. UpToDate. Waltham, MA: Wolters Kluwer; [cited 2025 Mar 23]. Available from: https://www.uptodate.com
- Spanish Ministry of Health. National Newborn Screening Program for Congenital Diseases [Internet]. Madrid: Ministry of Health; [cited 2025 Mar 23].
- Mohamed S, Kambal MA, Al Jurayyan NA, Al Nemri A, Babiker A, Hasanato R, et al. Tyrosinemia type 1: a rare and forgotten cause of reversible hypertrophic cardiomyopathy in infancy. BMC Res Notes. 2013;6:362. Available from: https://doi.org/10.1186/1756-0500-6-362
- Chinsky JM, Singh R, Ficicioglu C, van Karnebeek CDM, Grompe M, Mitchell G, et al. Diagnosis and treatment of tyrosinemia type I: a US and Canadian consensus group review and recommendations. Genet Med. 2017;19(12):1380–95. Available from: https://doi.org/10.1038/gim.2017.101
- Imseis EM, Bynon JS, Thornhill C. Case of hepatocellular carcinoma in a patient with hereditary tyrosinemia in the post-newborn screening era. World J Hepatol. 2017;9(9):487–90. Available from: https://doi.org/10.4254/wjh.v9.i9.487
- Nitisinone: new drug. Type 1 tyrosinemia: an effective drug. Prescrire Int. 2007;16(88):56–8. Available from: https://pubmed.ncbi.nlm.nih.gov/17458044/
- van Ginkel WG, Jahja R, Huijbregts SCJ, Daly A, MacDonald A, De Laet C, et al. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J Rare Dis. 2016;11:87. Available from: https://doi.org/10.1186/s13023-016-0472-5